Archive: 2026/02

Physical Therapy for Pain: Exercise, Stretching, and Restoration
- by Colin Edward Egan
- on 23 Feb 2026
Physical therapy for pain uses exercise, stretching, and movement to reduce pain naturally-without drugs. Proven methods like tai chi, two-minute routines, and graded activity help millions cut pain by half in just weeks. Learn how to start today.
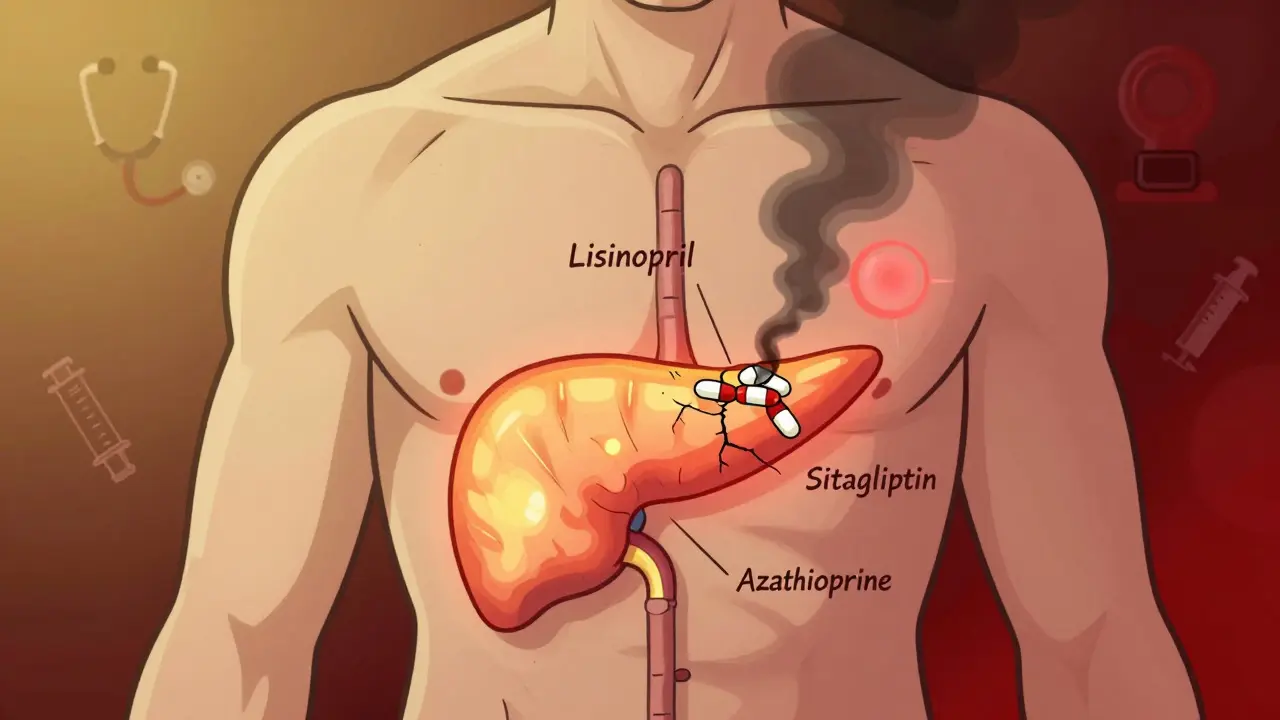
Severe Pancreatitis from Medications: Warning Signs and What to Do
- by Colin Edward Egan
- on 20 Feb 2026
Drug-induced severe pancreatitis is rare but deadly. Know the warning signs, which medications raise your risk, and how to act fast before it's too late. Early detection can mean the difference between full recovery and life-threatening complications.
Provider Case Studies: Real-World Experiences with Generic Medications
- by Colin Edward Egan
- on 19 Feb 2026
Providers share real-world experiences with generic medications, revealing that while most generics are safe and effective, substitution challenges persist-especially for drugs with narrow therapeutic indexes. Patient trust, state laws, and manufacturer changes all play critical roles.
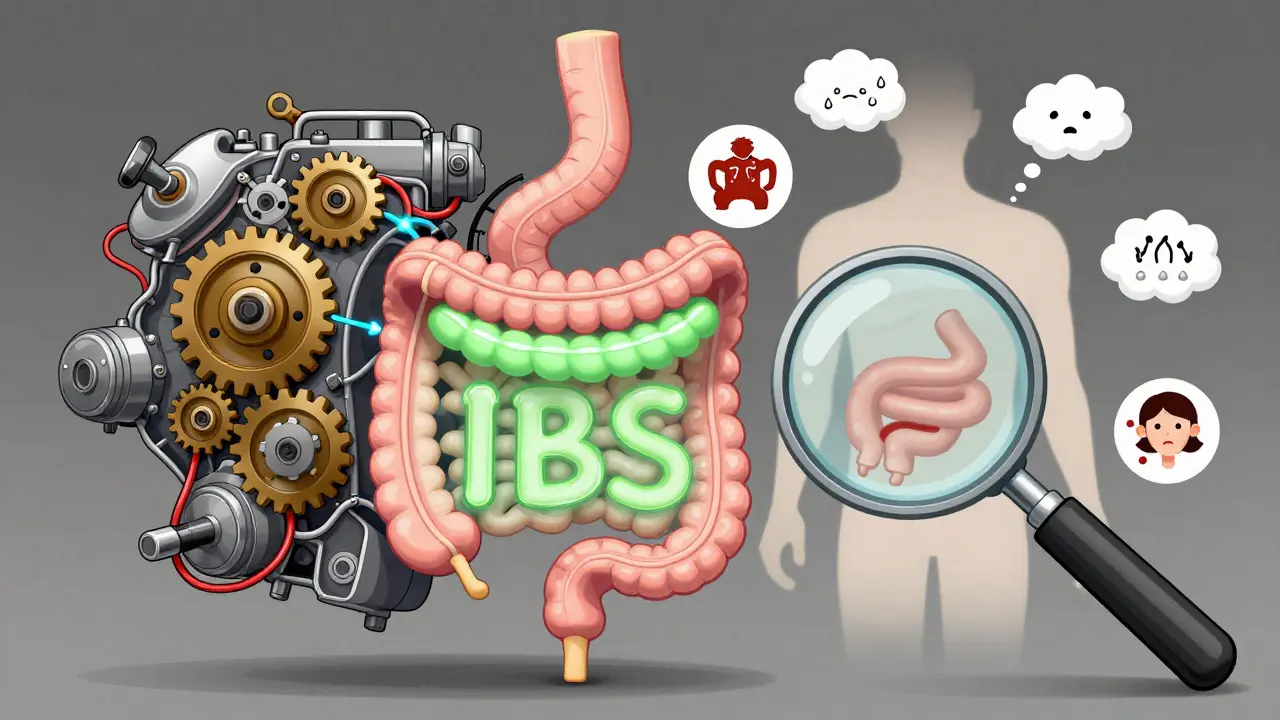
IBS vs. IBD: Functional vs. Inflammatory Bowel Disorders Explained
- by Colin Edward Egan
- on 16 Feb 2026
IBS and IBD share symptoms like bloating and diarrhea, but one is a functional disorder with no physical damage, while the other causes chronic inflammation and structural harm. Know the difference to get the right treatment.

Cost-Effectiveness Analysis: Measuring the Value of Generic Drugs
- by Colin Edward Egan
- on 13 Feb 2026
Cost-effectiveness analysis reveals that many generic drugs are priced far above their true value. By comparing real-world pricing and therapeutic alternatives, we can save billions-and ensure patients get the best care at the lowest cost.
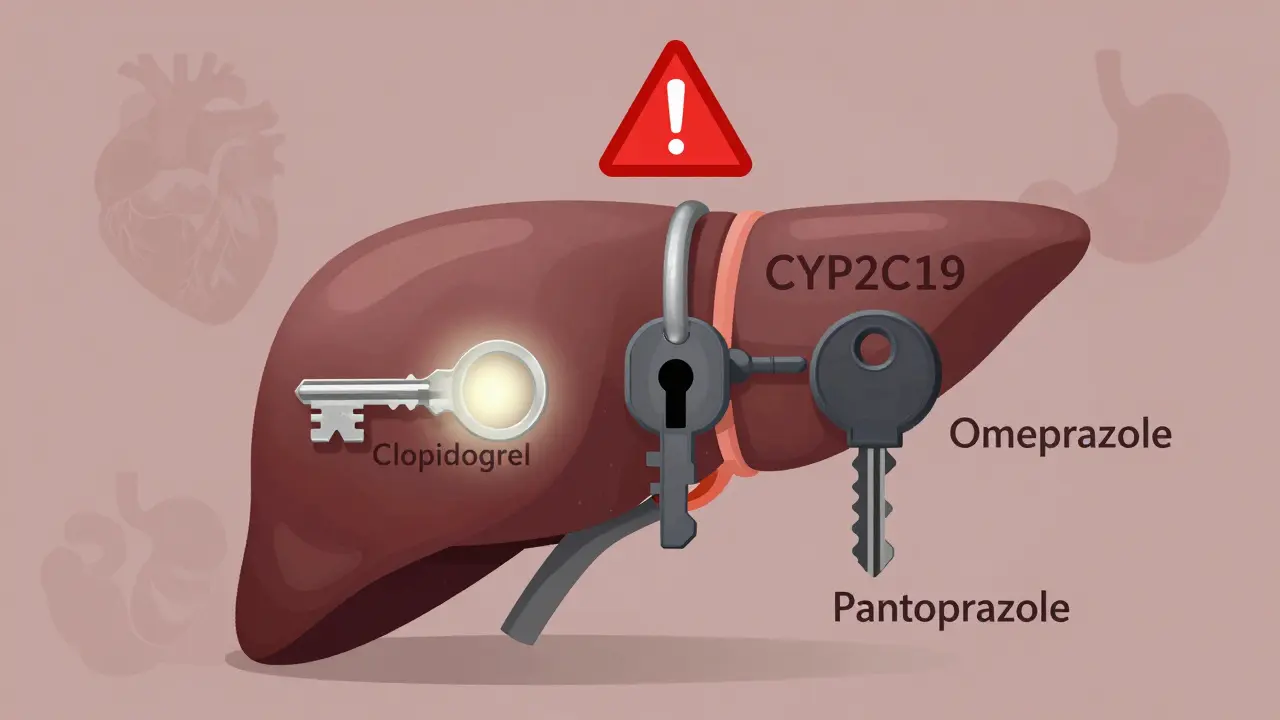
Proton Pump Inhibitors and Clopidogrel: What You Need to Know About the Drug Interaction
- by Colin Edward Egan
- on 12 Feb 2026
Clopidogrel and proton pump inhibitors like omeprazole can interact, reducing clopidogrel’s effectiveness and raising heart attack risk. Pantoprazole is the safest PPI choice. Learn why, who’s at risk, and what to do.
Red Yeast Rice and Statins: Why Combining Them Can Be Dangerous
- by Colin Edward Egan
- on 9 Feb 2026
Red yeast rice contains the same active ingredient as statins and can cause dangerous muscle and liver damage when taken together. Learn why combining them is risky - and what safer alternatives exist.

Fentanyl Patch Side Effects: Overdose and Withdrawal Risks Explained
- by Colin Edward Egan
- on 6 Feb 2026
Fentanyl patches treat severe chronic pain but carry serious risks. Learn about overdose symptoms like slow breathing and cold skin, withdrawal signs such as restlessness and nausea, and critical safety steps. Always follow medical advice to avoid life-threatening complications.
How to Use a Pill Organizer Safely Without Overdosing
- by Colin Edward Egan
- on 4 Feb 2026
Learn how to use a pill organizer correctly to prevent accidental overdoses. This guide covers common mistakes, proper filling steps, storage tips, and handling 'as needed' medications with expert-backed strategies.
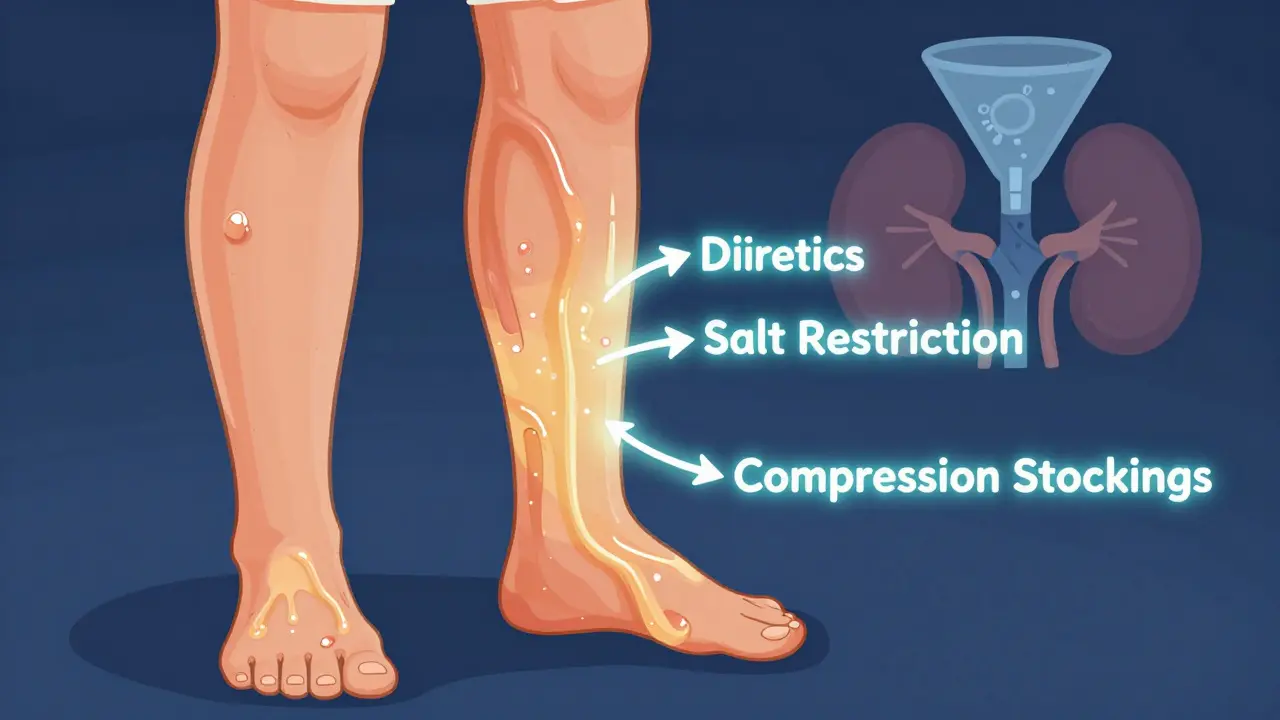
Edema in CKD: How Diuretics, Salt Restriction, and Compression Therapy Work Together
- by Colin Edward Egan
- on 3 Feb 2026
Edema in chronic kidney disease is caused by fluid buildup from poor sodium regulation. Diuretics, strict salt restriction, and compression therapy work together to reduce swelling, prevent complications, and improve quality of life. Learn how each method works-and why combining them is essential.
