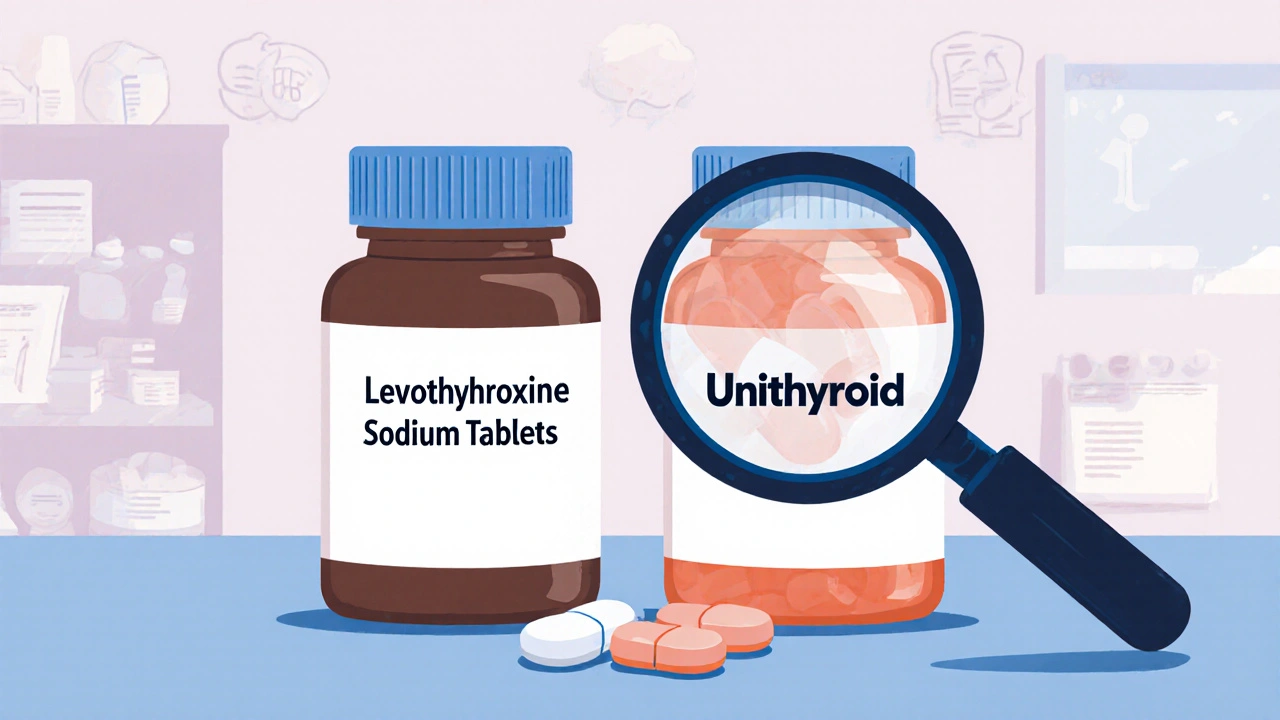
Have you ever picked up a prescription and noticed the pill looks exactly like your brand-name drug-but the box says something totally different? Maybe it’s labeled with a plain name like levothyroxine instead of Unithroid. You might wonder: Is this a fake? A cheaper version? Or something else entirely? This isn’t a mistake. It’s an authorized generic.
What Exactly Is an Authorized Generic?
An authorized generic is the exact same medication as the brand-name drug you’re used to-same active ingredient, same inactive ingredients, same shape, same strength, same manufacturer. The only difference? It doesn’t carry the brand name on the label. It’s sold under a generic name, often at a lower price, but it comes straight from the original drug company. The U.S. Food and Drug Administration (FDA) defines it clearly: an approved brand-name drug marketed without the brand name on the label. That’s it. No changes to the formula. No shortcuts in production. It’s identical in every way, except for the packaging and name. This isn’t a traditional generic. Traditional generics are made by other companies after the brand patent expires. They must prove they work the same way through clinical tests. Authorized generics skip that step because they’re made by the brand company itself-or under license by another company using the exact same formula and production line.How Are Authorized Generics Different From Regular Generics?
This is where things get confusing. Most people think all generics are the same. They’re not. Traditional generics must meet FDA standards for bioequivalence. That means they have the same active ingredient and work the same way in your body. But they can have different fillers, dyes, or coatings. Sometimes, patients report differences in how they feel-stomach upset, different absorption rates, or even allergic reactions to an inactive ingredient that wasn’t in the brand version. Authorized generics don’t have that problem. Since they’re made on the same line, with the same recipe, they’re chemically and physically identical to the brand-name drug. If you’ve ever had trouble switching to a generic because it “didn’t feel right,” an authorized generic might be the solution. Here’s a quick comparison:| Feature | Authorized Generic | Traditional Generic |
|---|---|---|
| Manufacturer | Same as brand-name drug (or licensed by them) | Separate company |
| Active Ingredient | Identical | Identical |
| Inactive Ingredients | Identical to brand | May differ |
| Approval Process | Uses brand’s NDA; no separate FDA approval needed | Requires ANDA; must prove bioequivalence |
| Listed in FDA Orange Book? | No | Yes |
| Typical Price | 15-30% lower than brand | Often 50-80% lower than brand |
Why Do Drug Companies Make Authorized Generics?
At first glance, it seems odd. Why would a company that spent millions developing a drug turn around and sell a copy of it at a lower price? The answer is business strategy. When a brand-name drug’s patent expires, other companies can legally make generic versions. But the brand company doesn’t want to lose all its customers overnight. So they launch their own “generic” version-authorized generic-to keep market share. It’s not charity. It’s survival. Research from Health Affairs found that between 2010 and 2019, pharmaceutical companies launched 854 authorized generics. In 75% of cases, they were introduced after traditional generics had already entered the market. That’s not random. It’s calculated. Often, the brand company waits until the first generic manufacturer gets the 180-day exclusivity period granted under the Hatch-Waxman Act. Then they roll out their authorized generic to split the market. The result? The first generic company doesn’t get to dominate. The brand company still gets revenue. And patients get a cheaper option. Some critics argue this delays true competition. Instead of letting multiple generic makers drive prices down, the brand company uses its own version to hold prices higher than they might otherwise be.How Do You Know If You’re Getting an Authorized Generic?
You won’t always know. Pharmacies often don’t tell you unless you ask. But there are clues. First, check the label. If the drug name matches the brand name’s active ingredient but the manufacturer is the same as the brand (e.g., Pfizer, AbbVie, Johnson & Johnson), it’s likely an authorized generic. Second, look at the pill. If it looks exactly like your brand pill-same color, same imprint, same size-but the box says something like “Levothyroxine Sodium Tablets” instead of “Unithroid,” it’s probably an authorized generic. Third, ask your pharmacist. They can check the National Drug Code (NDC) or contact the distributor. The FDA also maintains a public list of authorized generics, updated as of October 10, 2025. You can find it on their website under “Approved Drug Products with Therapeutic Equivalence Evaluations.”
Real Examples of Authorized Generics
Here are some common medications you might be taking that have authorized generic versions:- Colcrys (colchicine) → Authorized generic by Prasco Laboratories
- Concerta (methylphenidate ER) → Authorized generic by Watson/Actavis
- Celebrex (celecoxib) → Authorized generic by Greenstone Pharmaceuticals
- Unithroid (levothyroxine) → Authorized generic by Jerome Stevens Pharmaceuticals
- Viagra (sildenafil) → Authorized generic by Pfizer
Do Authorized Generics Save You Money?
Yes-but not always as much as you’d hope. Authorized generics usually cost 15% to 30% less than the brand-name version. That’s a real savings. But once multiple traditional generics enter the market, prices can drop even further-sometimes 80% below brand price. So if you’re paying $120 for a brand-name drug and your authorized generic costs $90, that’s a good deal. But if a traditional generic is selling for $15, you’re better off switching. The key is timing. Authorized generics often appear right after patent expiry, before other generics flood the market. If you catch them early, you save money. If you wait, you might miss the window.What Do Patients and Doctors Say?
Patients often don’t notice a difference-because there isn’t one. But some get confused. They’ve been told generics are “just as good,” then they get a pill that looks exactly like the brand but costs less. They wonder: Is this safe? Is this the real thing? Pharmacists report that patient education is the biggest challenge. Many people assume “generic” means “different.” But with authorized generics, that’s not true. They’re the same drug, just unlabeled. Doctors are divided. Some prefer authorized generics for patients who’ve had bad reactions to traditional generics. Others worry they’re a marketing tactic that keeps drug prices artificially high. Dr. Aaron Kesselheim, a Harvard Medical School researcher who studied authorized generics, put it bluntly: “Marketing appears strategic. In markets with traditional generics, three-fourths of authorized generics launched after the respective generic competition started.” In other words: They’re not here to help you. They’re here to help the company keep its profits.
Are Authorized Generics Safe?
Absolutely. Since they’re made by the same company, under the same quality controls, and use the exact same formula, they’re as safe as the brand-name drug. The FDA doesn’t require additional testing because they’re not a new product. They’re the same product with a different label. If the brand drug is safe, the authorized generic is too. The only risk? If you’re allergic to an inactive ingredient, and your brand drug and authorized generic use different ones-but that’s rare, because they usually use the same ones.What Should You Do?
If you’re on a brand-name drug that’s about to go generic:- Ask your pharmacist: “Is there an authorized generic for this?”
- Check your prescription label. Does the manufacturer match the brand company?
- Compare prices. If the authorized generic is cheaper than your brand, switch.
- If a traditional generic is much cheaper, go with that.
- Don’t assume all generics are the same. Ask for specifics.
Final Thought: It’s Not About Brand. It’s About Control.
Authorized generics blur the line between brand and generic. They’re a product of the modern pharmaceutical system-where companies don’t just sell drugs. They sell control. They’re not evil. They’re not scams. But they’re not purely altruistic either. Understanding them gives you power. You can choose the best option for your health-and your wallet.Are authorized generics the same as the brand-name drug?
Yes. Authorized generics are made by the same manufacturer using the exact same formula, including active and inactive ingredients, as the brand-name drug. The only difference is the label-they don’t carry the brand name.
Are authorized generics cheaper than traditional generics?
Usually not. Authorized generics are typically 15-30% cheaper than the brand-name version, but traditional generics often drop to 50-80% lower after multiple manufacturers enter the market. Authorized generics are priced to compete with the brand, not with other generics.
Why aren’t authorized generics listed in the FDA’s Orange Book?
The Orange Book only lists traditional generics that went through the Abbreviated New Drug Application (ANDA) process. Authorized generics are marketed under the original brand’s New Drug Application (NDA), so they don’t need separate approval and aren’t included in the list.
Can I trust an authorized generic if it looks different from my brand pill?
Yes. Authorized generics may have different colors, shapes, or imprints to distinguish them from the brand version, but they contain the exact same ingredients and dosage. The FDA requires this to prevent confusion. Always check the NDC code or ask your pharmacist if unsure.
Do authorized generics have the same side effects as the brand-name drug?
Yes. Since they are chemically identical, authorized generics have the same side effect profile as the brand-name drug. If you tolerated the brand well, you’ll likely tolerate the authorized generic just as well.

Kihya Beitz
November 14, 2025 AT 23:15So let me get this straight - the drug company makes the exact same pill, just removes the fancy logo, and calls it a 'generic' to charge you less? Bro, that’s not capitalism, that’s a magic trick with a prescription pad.
Jennifer Walton
November 16, 2025 AT 21:29Identity is a label. The pill doesn’t care what you call it.
Shyamal Spadoni
November 18, 2025 AT 01:02ok so here’s the real deal - big pharma dont wanna lose money so they make a fake generic to scare the real generics away like some kinda corporate ghost. and the fda? they just sit there like a bored librarian. and you think you’re saving money but really you’re just feeding the beast with a cheaper receipt. i saw a guy on reddit say he got the same pill from a mexican pharmacy for $3 and it was literally identical - same imprints, same taste even. so why are we paying $90? who’s really holding the bag here? the system is rigged and the pills are just the packaging.
Ogonna Igbo
November 19, 2025 AT 09:31Listen here America you think you own medicine? We in Nigeria know the truth - when your people get sick they beg for pills from India or China because your system is built to bleed the poor. You call this 'authorized generic'? It's just another tax on your suffering. Your drug companies don't make medicine - they make profit machines. And you call it healthcare? Ha. We have real generics that work and cost less than your coffee. You need to wake up.
BABA SABKA
November 21, 2025 AT 03:23Authorized generics are a tactical maneuver in the pharmaceutical arms race. They’re not about patient welfare - they’re about market share retention via regulatory arbitrage. The NDA-based distribution model bypasses ANDA competition, effectively creating a monopolistic pseudo-generic that suppresses price erosion. It’s predatory compliance. You’re not getting a discount - you’re getting a controlled reduction in choice architecture. The FDA’s Orange Book exclusion is a deliberate opacity mechanism. This isn’t innovation. It’s rent-seeking dressed in white lab coats.
Chris Bryan
November 21, 2025 AT 19:29They’re lying to you. This is how the globalists control your health. The same pills, same factory - but now they’re pushing you to switch so they can track you through your prescriptions. Your data is being sold. Your body is the product. Don’t be fooled by the label. Ask yourself - who really owns your medicine?
Jonathan Dobey
November 23, 2025 AT 01:50It’s like buying a Ferrari - but the dealer says, 'Hey, this is the exact same car, just without the prancing horse on the hood. Also, it’s 20% cheaper.' You’d think, 'Wait, did they just sell me my own damn car back to me?' That’s the emotional whiplash of authorized generics. The brand didn’t fade - it just went incognito. And we’re supposed to be grateful? This isn’t healthcare. It’s performance art for the pharmacoeconomic stage.
ASHISH TURAN
November 25, 2025 AT 01:06My dad switched to an authorized generic for his thyroid meds after he had bad reactions to the regular one. Said it felt like his old brand again - no stomach issues, no weird fatigue. He’s been on it for 3 years now. Just wanted to say - if you’ve had problems with generics before, this might actually help. Not all generics are equal. This one’s the real deal.
Ryan Airey
November 25, 2025 AT 19:58Stop pretending this is about patient care. This is corporate sabotage disguised as savings. The moment a generic hits the market, the brand drops its own version to kill competition before prices drop too far. This isn’t transparency - it’s market manipulation with a prescription. And you’re all falling for it like sheep.
Hollis Hollywood
November 26, 2025 AT 07:17I just want to say - I’ve been on levothyroxine for 12 years. I’ve tried three different generics. One made me dizzy. One gave me heart palpitations. The authorized generic? It felt like the brand again. No weird side effects. No guessing. I didn’t know what it was until my pharmacist told me. I was scared at first - like, is this safe? But it’s literally the same pill. Just cheaper. And honestly? I wish more people knew this existed. It’s not magic. It’s just medicine without the marketing hype. If you’ve struggled with generics before - don’t give up. Ask for the authorized one. It might be the missing piece.