Tag: generic equivalence
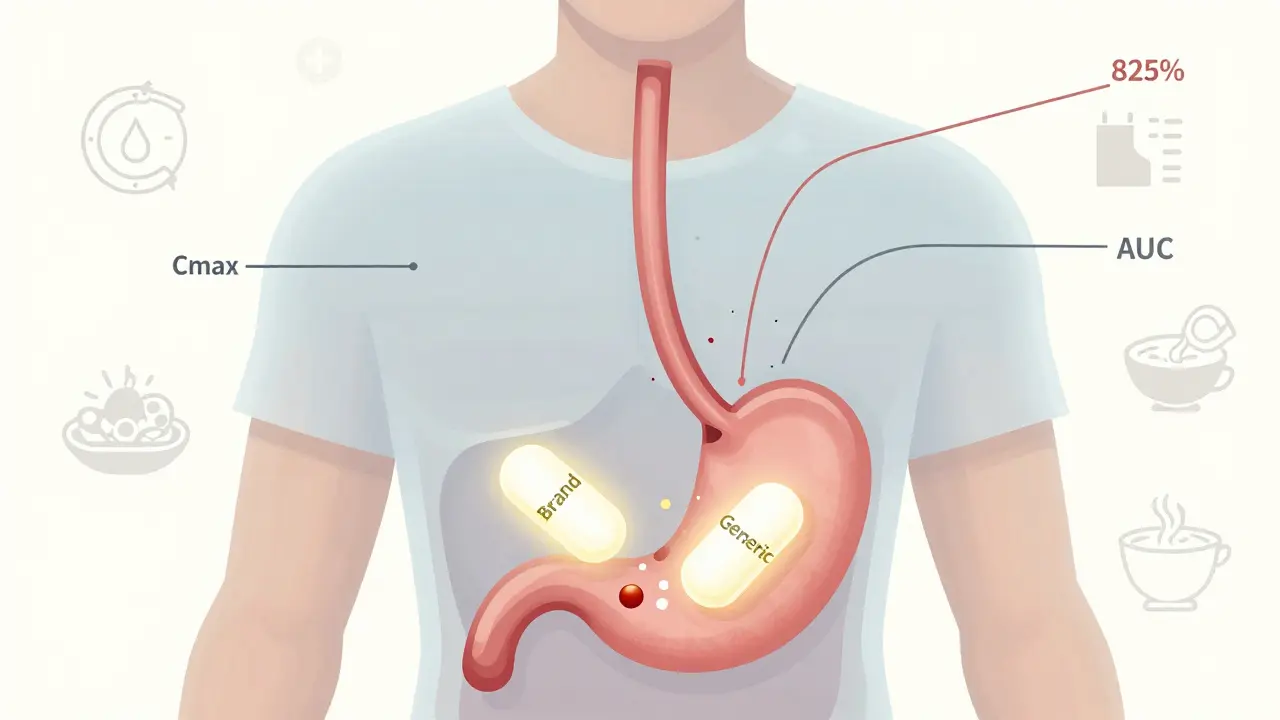
Pharmacokinetic Studies: The Real Standard for Proving Generic Drug Equivalence
- by Colin Edward Egan
- on 26 Jan 2026
Pharmacokinetic studies are the primary method used to prove generic drugs are equivalent to brand-name versions, but they're not perfect. Learn how they work, where they fall short, and what's changing in drug approval standards.

How Pharmacists Verify Generic Equivalence: Practice Standards
- by Colin Edward Egan
- on 21 Jan 2026
Pharmacists use the FDA's Orange Book to legally verify that generic drugs are therapeutically equivalent to brand-name medications. This process ensures safe, effective substitutions across the U.S., backed by bioequivalence data, state laws, and strict standards.
